Consider the equations below.
CH4 (g)->C(s)+2H2(g) H1 = 74.6 kJ
C(s) + 2CI2(g)->CC...

Chemistry, 28.01.2021 06:40 zoelynn8386
Consider the equations below.
CH4 (g)->C(s)+2H2(g) H1 = 74.6 kJ
C(s) + 2CI2(g)->CCI4(g) H2 = -95.7 kJ
2H2(g) + 2CI2(g)-> 4HCI(g) H3 = -184.6 kJ
CH4(g) + 4CI2(g) -> CCI4(g) + 4HCI(g) H4 = -205.7 kJ
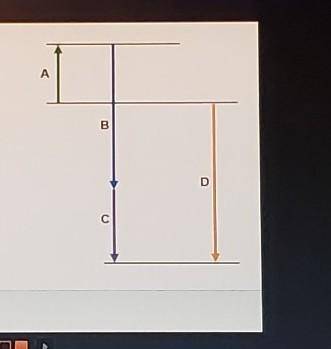
Complete the following based on the diagram.
Arrow A: 74.6 kJ
-95.7 kJ
-184.6 kJ
Arrow B: endothermic
exothermic
Arrow C: - bas a magnitude that is greater than that of B
- has a magnitude that is less than that of B
- has negative enthalpy
Arrow D: - represents an intermediate reaction
- has a magnitude that is always higher than any intermediate reaction
- represents the overall enthalpy of reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
Questions


Mathematics, 22.04.2020 03:41

Mathematics, 22.04.2020 03:41


Chemistry, 22.04.2020 03:41

History, 22.04.2020 03:41


History, 22.04.2020 03:41






History, 22.04.2020 03:41

Physics, 22.04.2020 03:41







