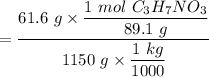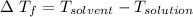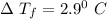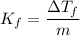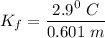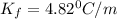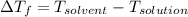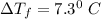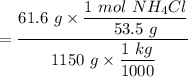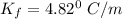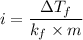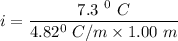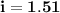Chemistry, 26.01.2021 05:30 christophercordero15
When 61.6 g of alanine (C3H7NO2) are dissolved in 1150. g of a certain mystery liquid X, the freezing point of the solution is 2.9 °C lower than the freezing point of pure X. On the other hand, when 61.6 g of ammonium chloride (NH4CI) are dissolved in the same mass of X. The freezing point of the solution is 7.3 °C lower than the freezing point of pure X Calculate the van't Hoff factor for ammonium chloride in X.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
When 61.6 g of alanine (C3H7NO2) are dissolved in 1150. g of a certain mystery liquid X, the freezin...
Questions






Mathematics, 18.10.2020 16:01


History, 18.10.2020 16:01


Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Arts, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01




Mathematics, 18.10.2020 16:01