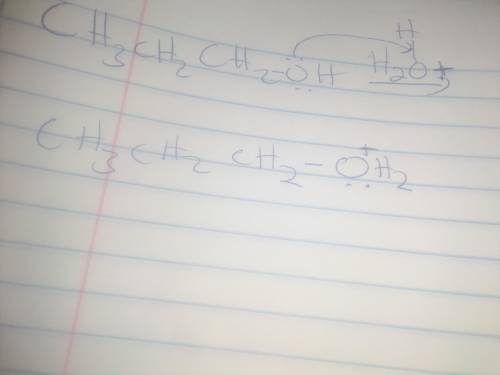When propyl alcohol is treated with acid, the initially formed intermediate is known as an oxonium ion. There is a scheme of a reversible chemical reaction. The substrates are CH3CH2CH2OH molecule and H with a charge of 1 plus ion. The product is CH3CH2CH2OH2 with a charge of 1 plus ion. Oxygen atom in CH3CH2CH2OH molecule has 2 lone pairs. Oxygen atom in CH3CH2CH2OH2 with a charge of 1 plus ion has a lone pair. All bonds are single. Using the curved arrow formalism, show how this process most likely occurs.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
When propyl alcohol is treated with acid, the initially formed intermediate is known as an oxonium i...
Questions


Physics, 24.04.2020 19:53




English, 24.04.2020 19:53


Spanish, 24.04.2020 19:53

History, 24.04.2020 19:53

History, 24.04.2020 19:54





Physics, 24.04.2020 19:54

Social Studies, 24.04.2020 19:54

Social Studies, 24.04.2020 19:54



History, 24.04.2020 19:54