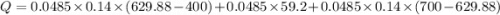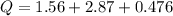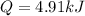Chemistry, 11.01.2021 16:30 valeriegarcia12
How much heat is required to change 48.5 liquid mercury (Hg) at 400 K to vapor at a 700 K? The boiling point of mercury is 629.88 K
-88 kj
-204 kj
16.2 kj
30 kj
29 kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
How much heat is required to change 48.5 liquid mercury (Hg) at 400 K to vapor at a 700 K? The boili...
Questions

Mathematics, 10.06.2021 01:50


Mathematics, 10.06.2021 02:00




Mathematics, 10.06.2021 02:00

Mathematics, 10.06.2021 02:00




Chemistry, 10.06.2021 02:00


Mathematics, 10.06.2021 02:00

Mathematics, 10.06.2021 02:00


Mathematics, 10.06.2021 02:00

Chemistry, 10.06.2021 02:00


Mathematics, 10.06.2021 02:00

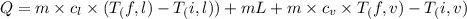

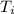 = 400 K
= 400 K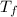 = 700 K
= 700 K