
Chemistry, 11.01.2021 14:00 okitsfrizz6366
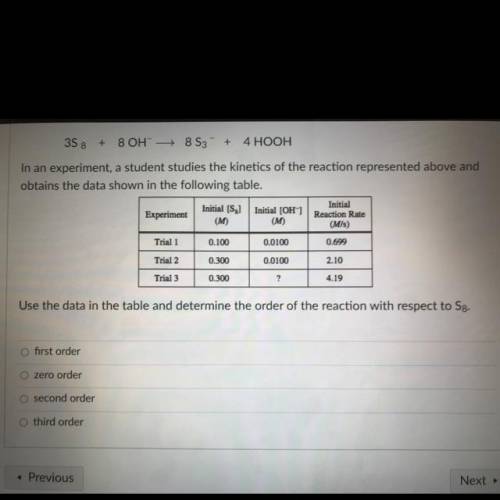
3S8 + 8 OH- + 8 S3 + 4 HOOH
In an experiment, a student studies the kinetics of the reaction represented above and
obtains the data shown in the following table.
Initial [S
Initial
Initial (OH)
Experiment
Reaction Rate
(M)
(M)
(M/s)
Trial 1
0.100
0.0100
0.699
Trial 2
0.300
0.0100
2.10
Trial 3
0.300
?
4.19
Use the data in the table and determine the order of the reaction with respect to Sg.
O first order
O zero order
O second order
O third order

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1
You know the right answer?
3S8 + 8 OH- + 8 S3 + 4 HOOH
In an experiment, a student studies the kinetics of the reaction repres...
Questions

Mathematics, 24.09.2019 07:30


Mathematics, 24.09.2019 07:30





English, 24.09.2019 07:30


Mathematics, 24.09.2019 07:30



Mathematics, 24.09.2019 07:30


Biology, 24.09.2019 07:30



English, 24.09.2019 07:30


English, 24.09.2019 07:30



