Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1
You know the right answer?
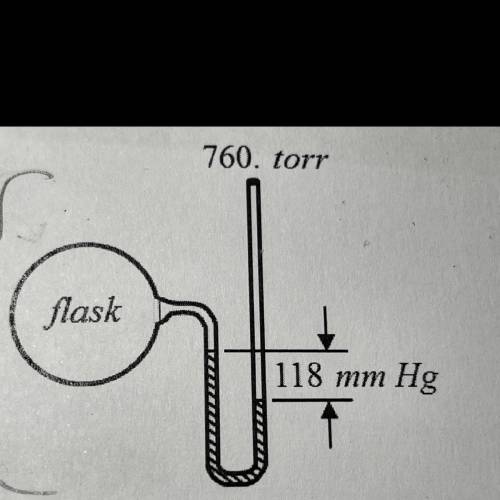
mercury’s density is 13.6 g/mL. now assume that the manometer shown above contains an oil density 1....
Questions




History, 06.11.2019 22:31

Chemistry, 06.11.2019 22:31





Biology, 06.11.2019 22:31






Mathematics, 06.11.2019 22:31

Business, 06.11.2019 22:31



Business, 06.11.2019 22:31