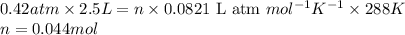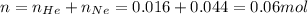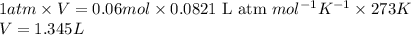Chemistry, 26.11.2019 14:31 meganwintergirl
A2.5l flask at 15°c contains a mixture of n2, he and ne at partial pressure of 0.32 atm for n2, 0.15 atm for he and 0.42 atm for ne. calculate the volume in liter at stp occupied by he and ne if the n2 is removed selectively. how to solve this problem?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1


Chemistry, 23.06.2019 11:00
Just on number 2 (all parts), and if you do answer explain in detail
Answers: 3
You know the right answer?
A2.5l flask at 15°c contains a mixture of n2, he and ne at partial pressure of 0.32 atm for n2, 0.15...
Questions

Social Studies, 14.02.2020 23:52



Chemistry, 14.02.2020 23:52



Mathematics, 14.02.2020 23:52



Chemistry, 14.02.2020 23:52










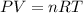 .......(1)
.......(1)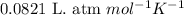
![P=0.15atm\\V=2.5L\\T=15^oC=[15+273]=288K\\n=?](/tpl/images/0391/5646/f57b5.png)
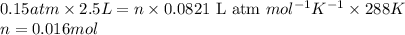
![P=0.42atm\\V=2.5L\\T=15^oC=[15+273]=288K\\n=?](/tpl/images/0391/5646/48efc.png)