
Chemistry, 11.01.2021 03:40 offensiveneedle
Using the following equation: 3H2SO4 + 2Fe - +Fe2(SO4)3 + 3H2 How many moles of H2SO4 will react with 8 moles of Fe?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

You know the right answer?
Using the following equation: 3H2SO4 + 2Fe - +Fe2(SO4)3 + 3H2
How many moles of H2SO4 will react wi...
Questions


Biology, 20.02.2020 17:51


Computers and Technology, 20.02.2020 17:51


History, 20.02.2020 17:51


Computers and Technology, 20.02.2020 17:51








Mathematics, 20.02.2020 17:52




Chemistry, 20.02.2020 17:52

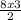 = 12 moles of H₂SO₄
= 12 moles of H₂SO₄

