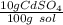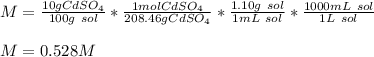Chemistry, 08.01.2021 17:10 danteyoungblood7
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol) by mass? The density of the solution is 1.10 g/mL.
a) 0.528 M
b) 0.436 M
c) 0.479 M
d) 0.048 M
e) 22.9 M

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol)...
Questions

Physics, 14.07.2020 01:01

English, 14.07.2020 01:01





Chemistry, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01


Computers and Technology, 14.07.2020 01:01





Mathematics, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01


Biology, 14.07.2020 01:01