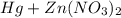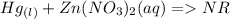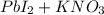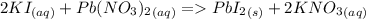USE LETTERS FOR ANSWERS, NO NEED TO EXPLAIN
a) Substitute symbols and formulas for words.
b...

USE LETTERS FOR ANSWERS, NO NEED TO EXPLAIN
a) Substitute symbols and formulas for words.
b) Predict the products. If no reaction occurs, write “NR” after the --> sign. c) Include abbreviations for the physical state.
d) Write out and balance the equation.
6. Mercury is poured into an aqueous solution of zinc nitrate.
7. Solutions of potassium iodide and lead (II) nitrate are mixed. The resulting lead compound is a precipitate.
thank you! :)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2


Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1
You know the right answer?
Questions




Mathematics, 05.10.2019 18:10


History, 05.10.2019 18:10


Chemistry, 05.10.2019 18:10

Mathematics, 05.10.2019 18:10


History, 05.10.2019 18:10

History, 05.10.2019 18:10

Mathematics, 05.10.2019 18:10




English, 05.10.2019 18:10

World Languages, 05.10.2019 18:10

Mathematics, 05.10.2019 18:10