
Chemistry, 29.12.2020 14:00 CameronVand21
What would happen to the rate of a reaction with rate law rate = k [NO]^2[H2] if
the concentration of NO were halved?
A. The rate would be four times larger.
B. The rate would also be halved.
C. The rate would be one-fourth.
D. The rate would be doubled.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

You know the right answer?
What would happen to the rate of a reaction with rate law rate = k [NO]^2[H2] if
the concentration...
Questions

Social Studies, 15.12.2020 01:00

Biology, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00




World Languages, 15.12.2020 01:00



Mathematics, 15.12.2020 01:00





SAT, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

English, 15.12.2020 01:00

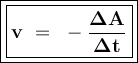
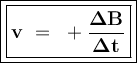
![\tt r_2=k[\dfrac{1}{2}No]^2[H_2]\\\\r_2=\dfrac{1}{4}k.[No]^2[H_2]\\\\r_2=\dfrac{1}{4}r_1](/tpl/images/1009/0129/44ffe.png)


