
Chemistry, 22.12.2020 01:00 ayoismeisjuam
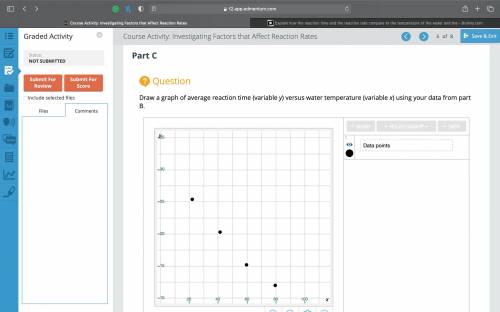
Explain how the reaction time and the reaction rate compare to the temperature of the water and the reactants. Revisit your hypothesis from part A, and explain how it compares to your experimental results. Study the trend of the graph from part C. What would the reaction time be (in seconds) if the water were cooled to 5°C?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

You know the right answer?
Explain how the reaction time and the reaction rate compare to the temperature of the water and the...
Questions





Mathematics, 16.11.2019 01:31

Mathematics, 16.11.2019 01:31




Mathematics, 16.11.2019 01:31

Physics, 16.11.2019 01:31

Mathematics, 16.11.2019 01:31

Chemistry, 16.11.2019 01:31



Mathematics, 16.11.2019 01:31


Computers and Technology, 16.11.2019 01:31

History, 16.11.2019 01:31




