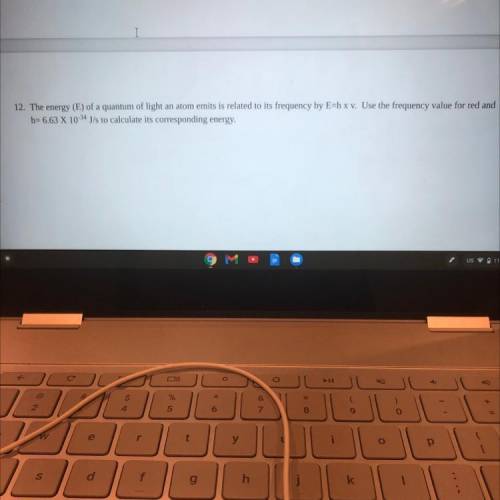
PLEASE QUICK IM ON A DEADLINE
...

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 13:00
Using the periodic table complete the table to describe each atom type in your answers
Answers: 1

Chemistry, 23.06.2019 19:30
Which of the following is true about science? select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1
You know the right answer?
Questions


Arts, 23.04.2021 20:10


Mathematics, 23.04.2021 20:10

Mathematics, 23.04.2021 20:10


History, 23.04.2021 20:10


Mathematics, 23.04.2021 20:10



Business, 23.04.2021 20:10


German, 23.04.2021 20:10

History, 23.04.2021 20:10


History, 23.04.2021 20:10

Arts, 23.04.2021 20:10

Mathematics, 23.04.2021 20:10