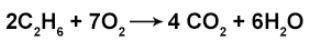How many moles of H2O are produced when 3.25 moles of O2 react in the above equation?
a
12.92...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2
You know the right answer?
Questions


History, 19.11.2019 13:31

Mathematics, 19.11.2019 13:31


Spanish, 19.11.2019 13:31

History, 19.11.2019 13:31


History, 19.11.2019 13:31

English, 19.11.2019 13:31


History, 19.11.2019 13:31

Mathematics, 19.11.2019 13:31

Mathematics, 19.11.2019 13:31

English, 19.11.2019 13:31

Geography, 19.11.2019 13:31

English, 19.11.2019 13:31



History, 19.11.2019 13:31