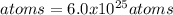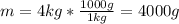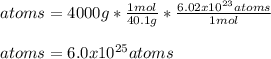Chemists use the mole as the unit for the amount of substance. One mole is equal to 6.02 x 10^23 (just like one dozen is equal to 12). A 40.1 g sample of calcium contains approximately 1 mole of calcium atoms. How many calcium atoms are in a 4 kg sample of calcium?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
Chemists use the mole as the unit for the amount of substance. One mole is equal to 6.02 x 10^23 (ju...
Questions


Mathematics, 09.06.2021 19:00


Mathematics, 09.06.2021 19:00

Mathematics, 09.06.2021 19:00

Mathematics, 09.06.2021 19:00

Mathematics, 09.06.2021 19:00



Mathematics, 09.06.2021 19:00



English, 09.06.2021 19:00




English, 09.06.2021 19:00

Mathematics, 09.06.2021 19:00


English, 09.06.2021 19:00