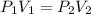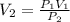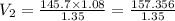Chemistry, 06.12.2020 08:30 Terrilady5
Air trapped in a cylinder fitted with piston occupies 145.7 mL at 1.08 atm pressure. What is the new volume when the piston is depressed, increasing the pressure to 1.35

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Air trapped in a cylinder fitted with piston occupies 145.7 mL at 1.08 atm pressure. What is the new...
Questions


Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30

Computers and Technology, 25.07.2019 05:30


English, 25.07.2019 05:30

English, 25.07.2019 05:30

History, 25.07.2019 05:30