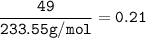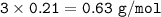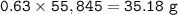Chemistry, 03.12.2020 14:20 bgallman153p71edg
In if the reaction below 49 grams of Fe3O4 produces a 78.25% yield of Fe. How many grams are produced? Fe3O4 + 4H2 = 3Fe + 4H2O Imagine the = is an arrow

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
In if the reaction below 49 grams of Fe3O4 produces a 78.25% yield of Fe. How many grams are produce...
Questions

Mathematics, 08.12.2020 03:50


English, 08.12.2020 03:50

Mathematics, 08.12.2020 03:50

Business, 08.12.2020 03:50

Mathematics, 08.12.2020 03:50


Computers and Technology, 08.12.2020 03:50

Geography, 08.12.2020 03:50


Biology, 08.12.2020 03:50


Mathematics, 08.12.2020 03:50

Chemistry, 08.12.2020 03:50

Mathematics, 08.12.2020 03:50


History, 08.12.2020 03:50

Geography, 08.12.2020 03:50


Spanish, 08.12.2020 03:50