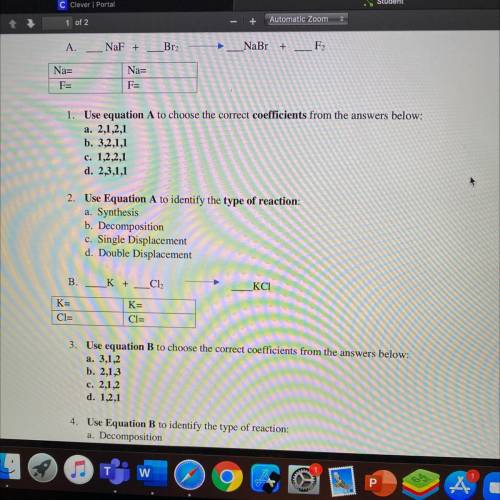
NaF +
A.
Br2
NaBr
+
F2
Na=
F=
Na=
F=
1. Use e...

Chemistry, 20.11.2020 22:10 bobbykemp300
NaF +
A.
Br2
NaBr
+
F2
Na=
F=
Na=
F=
1. Use equation A to choose the correct coefficients from the answers below:
a. 2,1,2,1
b. 3,2,1,1
c. 1,2,2,1
d. 2,3,1,1
2. Use Equation A to identify the type of reaction:
a. Synthesis
b. Decomposition
c. Single Displacement
d. Double Displacement
B.
K +
Cl2
КСІ
K=
CI=
K=
CI=
3. Use equation B to choose the correct coefficients from the answers below:
a. 3,1,2
b. 2,1,3
c. 2,1,2
d. 1,2,1

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Questions



English, 02.07.2019 06:10



Spanish, 02.07.2019 06:10
















