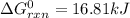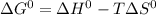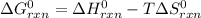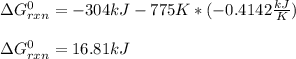Chemistry, 20.11.2020 14:00 camihecma1057
Estimate ΔG°rxn for the following reaction at 775 K. 2Hg(g) + O2(g) → 2HgO(s) ΔH°= -304.2 kJ; ΔS° = -414.2 J/K

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 23.06.2019 10:10
Which orbitals form a pi bond? a. the s orbital and three p orbitals b. the s orbital and two p orbitals c. overlapping p orbitals d. overlapping hybrid orbitals
Answers: 2
You know the right answer?
Estimate ΔG°rxn for the following reaction at 775 K. 2Hg(g) + O2(g) → 2HgO(s) ΔH°= -304.2 kJ; ΔS° =...
Questions



Mathematics, 03.07.2019 23:00



History, 03.07.2019 23:00


History, 03.07.2019 23:00


Mathematics, 03.07.2019 23:00


History, 03.07.2019 23:00


Mathematics, 03.07.2019 23:00

History, 03.07.2019 23:00





History, 03.07.2019 23:00