
Chemistry, 20.11.2020 14:00 bryson9604
What mass (in g) of lithium metal can be produced by the electrolysis of molten LiCl for 23.93 hr with an electrical current of 17.7?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1


Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
What mass (in g) of lithium metal can be produced by the electrolysis of molten LiCl for 23.93 hr wi...
Questions

History, 19.11.2020 01:00


Chemistry, 19.11.2020 01:00

Mathematics, 19.11.2020 01:00


Geography, 19.11.2020 01:00

Biology, 19.11.2020 01:00

Mathematics, 19.11.2020 01:00

Chemistry, 19.11.2020 01:00



Biology, 19.11.2020 01:00


Chemistry, 19.11.2020 01:00

English, 19.11.2020 01:00

History, 19.11.2020 01:00


Physics, 19.11.2020 01:00

English, 19.11.2020 01:00

Mathematics, 19.11.2020 01:00

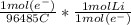 = 0.2634 mol Li
= 0.2634 mol Li

