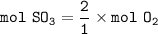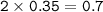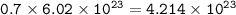Chemistry, 06.11.2020 23:30 claytonashley30
How many molecules of SO₃ can be formed from 0.35 moles of O₂ (assuming excess SO₂) from the following UNBALANCED equation? SO₂(g) + O₂(g) → SO₃(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
How many molecules of SO₃ can be formed from 0.35 moles of O₂ (assuming excess SO₂) from the followi...
Questions



Computers and Technology, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01


Chemistry, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

Chemistry, 20.09.2020 07:01


Biology, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

English, 20.09.2020 07:01



Mathematics, 20.09.2020 07:01