Chemistry, 06.11.2020 23:10 kokilavani
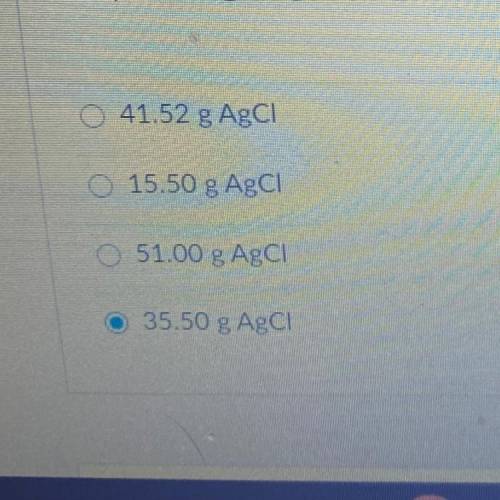
15.50 g of NH4Cl reacts with an excess of AgNO3. In the reaction 35.50 g
AgCl is produced. What is the percent yield of AgCl?
NH4Cl + AgNO3 --> AgCl + NH4NO3
69.61 % Yield AgCl
0 43.66 % Yield AgCl
85.50 % Yield AgCI
O 85.50 g AgCI

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1
You know the right answer?
15.50 g of NH4Cl reacts with an excess of AgNO3. In the reaction 35.50 g
AgCl is produced. What is...
Questions

Chemistry, 17.10.2021 02:00


Physics, 17.10.2021 02:00

World Languages, 17.10.2021 02:00



Mathematics, 17.10.2021 02:00



Biology, 17.10.2021 02:00





World Languages, 17.10.2021 02:00

Mathematics, 17.10.2021 02:00

Mathematics, 17.10.2021 02:00