
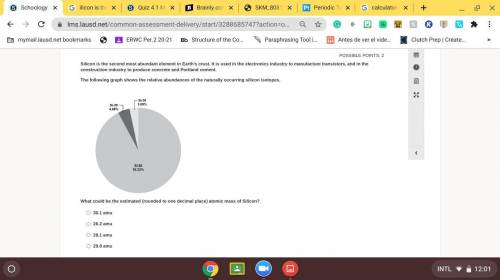
Silicon is the second most abundant element in Earth’s crust. It is used in the electronics industry to manufacture transistors, and in the construction industry to produce concrete and Portland cement. The following graph shows the relative abundances of the naturally occurring silicon isotopes.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
Silicon is the second most abundant element in Earth’s crust. It is used in the electronics industry...
Questions



Chemistry, 31.12.2021 01:00


Social Studies, 31.12.2021 01:00


Advanced Placement (AP), 31.12.2021 01:00




History, 31.12.2021 01:00






Social Studies, 31.12.2021 01:00

Biology, 31.12.2021 01:00


Social Studies, 31.12.2021 01:00



