
Chemistry, 03.11.2020 17:20 jaycobgarciavis
A chemist titrates of a aniline solution with solution at . Calculate the pH at equivalence. The of aniline is . Round your answer to decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of solution added.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1
You know the right answer?
A chemist titrates of a aniline solution with solution at . Calculate the pH at equivalence. The of...
Questions

Mathematics, 16.07.2020 21:01




History, 16.07.2020 21:01


Mathematics, 16.07.2020 21:01


Mathematics, 16.07.2020 21:01

Mathematics, 16.07.2020 21:01


Mathematics, 16.07.2020 21:01


Computers and Technology, 16.07.2020 21:01

English, 16.07.2020 21:01

Biology, 16.07.2020 21:01

Mathematics, 16.07.2020 21:01

Mathematics, 16.07.2020 21:01

Mathematics, 16.07.2020 21:01

Mathematics, 16.07.2020 21:01

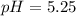
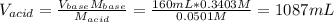
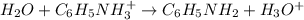
![K_a=\frac{[C_6H_5NH_2][H_3O^+]}{[C_6H_5NH_3^+]}](/tpl/images/0863/4824/b0008.png)
![Ka=\frac{K_w}{K_b}=\frac{1x10^-14}{1.35x10^{-5}}=7.41x10^{-10}\\\\7.41x10^{-10}=\frac{x*x}{[C_6H_5NH_3^+]_0}](/tpl/images/0863/4824/9b6f8.png)
![[C_6H_5NH_3^+]_0=\frac{0.16L*0.3403mol/L}{(1.087+0.16)L} =0.0437M](/tpl/images/0863/4824/b6b0e.png)
 is:
is:![[H_3O^+]=x=\sqrt{7.41x10^{-10}*0.0437M}=5.69x10^{-6}M](/tpl/images/0863/4824/79a1f.png)
![pH=-log([H_3O^+])=-log(5.67x10^{-6})\\\\pH=5.25](/tpl/images/0863/4824/95f39.png)


