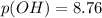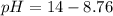A chemist titrates of a aniline solution with HCl solution at . Calculate the pH at equivalence. The of aniline is . Round your answer to decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of solution added.

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
The volume of a sphere is given by v= (4/3) pi r cubed, where r is the radius. compute the volume of a sphere with a radius of 117pm. state your answer in units of cubed.
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2
You know the right answer?
A chemist titrates of a aniline solution with HCl solution at . Calculate the pH at equivalence. The...
Questions

Mathematics, 05.08.2020 02:01


History, 05.08.2020 02:01


Mathematics, 05.08.2020 02:01


English, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01



Mathematics, 05.08.2020 02:01


Social Studies, 05.08.2020 02:01





Mathematics, 05.08.2020 02:01


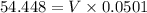
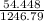 = 0.044 M
= 0.044 M![p(OH)=\frac{1}{2} [pKw+pKb+logC]](/tpl/images/0863/4823/b2809.png)
![p(OH)=\frac{1}{2} [14+4.87+log0.044]](/tpl/images/0863/4823/70b78.png)