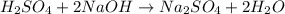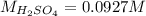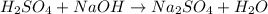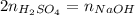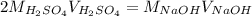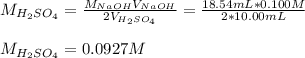Chemistry, 03.11.2020 09:40 kieranoid2017
4) 10.00 mL of sulfuric acid is neutralized in a titration using 18.54 mL of 0.100 M NaOH.
a) Write the neutralization equation for this reaction.
b) What is the concentration of the sulfuric acid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
4) 10.00 mL of sulfuric acid is neutralized in a titration using 18.54 mL of 0.100 M NaOH.
a) Write...
Questions

Mathematics, 16.04.2021 18:50

Mathematics, 16.04.2021 18:50

Mathematics, 16.04.2021 18:50




Mathematics, 16.04.2021 18:50


Health, 16.04.2021 18:50

Mathematics, 16.04.2021 18:50

Mathematics, 16.04.2021 18:50


Mathematics, 16.04.2021 18:50

Social Studies, 16.04.2021 18:50

Mathematics, 16.04.2021 18:50


Mathematics, 16.04.2021 18:50



Mathematics, 16.04.2021 18:50