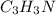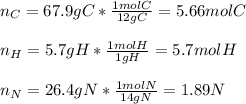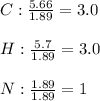Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Calculate the empirical formula of a compound that contains 67.9% carbon, 5.7 % H and 26.4 % N. When...
Questions







Mathematics, 25.01.2021 14:00

History, 25.01.2021 14:00

English, 25.01.2021 14:00

Chemistry, 25.01.2021 14:00

History, 25.01.2021 14:00

Physics, 25.01.2021 14:00


World Languages, 25.01.2021 14:00

Spanish, 25.01.2021 14:00

Mathematics, 25.01.2021 14:00


Chemistry, 25.01.2021 14:00

Geography, 25.01.2021 14:00

History, 25.01.2021 14:00