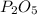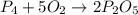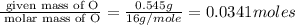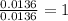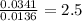Chemistry, 29.08.2019 07:50 jessezarate4513
When 0.422g of phosphorus is burned, 0.967g of a white oxide is obtained.
a. determine the empirical formula of the oxide
b. write a balanced equation for the reaction of phosphorus and molecular oxygen on the basis of this empirical formula

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
When 0.422g of phosphorus is burned, 0.967g of a white oxide is obtained.
a. determine the em...
a. determine the em...
Questions







Biology, 22.04.2020 01:44


Mathematics, 22.04.2020 01:44


History, 22.04.2020 01:44



Computers and Technology, 22.04.2020 01:44





Mathematics, 22.04.2020 01:45