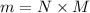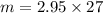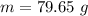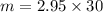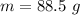Chemistry, 02.10.2020 17:01 trintrin227
Hydrogen cyanide, HCN, can be made by a two-step process. First, ammonia reacts with O2 to give nitric oxide, NO.
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
Then nitric oxide reacts with methane, CH4.
2NO(g) + 2CH4(g) → 2HCN(g) + 2H2O(g) + H2(g)
When 50.2 g of ammonia and 48.4 g of methane are used, how many grams of hydrogen cyanide can be produced? How many grams of which reactant remain at the end of both reactions? (You may assume that O2 is in excess in the first reaction.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What is the relationship between wind and ocean waves? question 17 options: wind moving at higher speeds will transfer more energy to the water, resulting in stronger waves. wind moving at higher speeds will transfer energy over a larger part of the ocean water, resulting in waves with a shorter wavelength. winds moving at higher speeds with cause water to move forward at faster rates, causing larger ocean waves. winds moving at higher speeds will affect deeper water, resulting in waves that move at a faster rate. how do temperature and salinity affect deepwater currents? question 15 options: as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 14:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 6.00 mol fe and 8.45 mol nio(oh) react?
Answers: 1
You know the right answer?
Hydrogen cyanide, HCN, can be made by a two-step process. First, ammonia reacts with O2 to give nitr...
Questions

Mathematics, 04.06.2020 12:57

Mathematics, 04.06.2020 12:57


Mathematics, 04.06.2020 12:57


Social Studies, 04.06.2020 12:57


Mathematics, 04.06.2020 12:57




English, 04.06.2020 12:57


Mathematics, 04.06.2020 12:57



Business, 04.06.2020 12:58



Mathematics, 04.06.2020 12:58

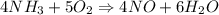
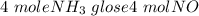
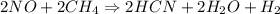
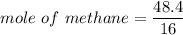
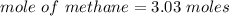
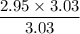 = 2.95 mol HCN
= 2.95 mol HCN