
Chemistry, 25.09.2020 09:01 rachel63892
LOL please help me ✨
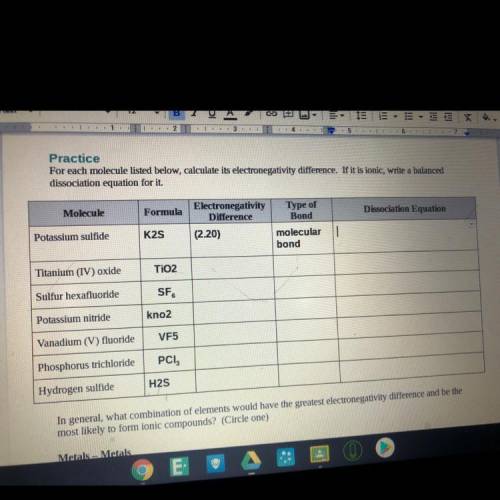
For each molecule listed below, calculate its electronegativity difference. If it is ionic, write a balanced
dissociation equation for it
Dissociation Equation
Molecule
Formula
Electronegativity
Difference
(2.20)
Type of
Bond
molecular
bond
Potassium sulfide
K2S
Titanium (IV) oxide
TiO2
1
Sulfur hexafluoride
SF
Potassium nitride
kno2
Vanadium (V) fluoride
VF5
Phosphorus trichloride
PCI,
Hydrogen sulfide
H2S

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1


You know the right answer?
LOL please help me ✨
For each molecule listed below, calculate its electronegativity difference. If...
Questions




Mathematics, 26.01.2021 21:50

Social Studies, 26.01.2021 21:50



Mathematics, 26.01.2021 21:50



Geography, 26.01.2021 21:50



English, 26.01.2021 21:50

English, 26.01.2021 21:50

Mathematics, 26.01.2021 21:50

Mathematics, 26.01.2021 21:50


German, 26.01.2021 21:50

English, 26.01.2021 21:50



