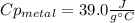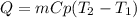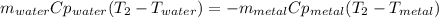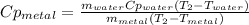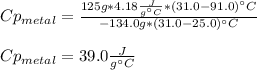Chemistry, 22.09.2020 04:01 jakiyahporter0817
A 134.0 g sample of an unknown metal is heated to 91.0⁰C and then placed in 125 g of water at 25.0⁰C. The final temperature of the water is measured at 31.0⁰C. Calculate the specific heat capacity of the unknown metal. Specific Heat of water is 4.18 J/g*C pls answer as quickly as possible

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3
You know the right answer?
A 134.0 g sample of an unknown metal is heated to 91.0⁰C and then placed in 125 g of water at 25.0⁰C...
Questions

History, 07.12.2021 21:30


Social Studies, 07.12.2021 21:30


Physics, 07.12.2021 21:30



English, 07.12.2021 21:30


Chemistry, 07.12.2021 21:30

Mathematics, 07.12.2021 21:30


Mathematics, 07.12.2021 21:30

Business, 07.12.2021 21:30


English, 07.12.2021 21:30

History, 07.12.2021 21:30



Biology, 07.12.2021 21:30