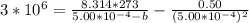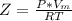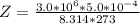Chemistry, 10.09.2020 04:01 llnapier8924
A certain gas obeys the van der Waals equation with a = 0.50 m6 Pa mol−2. Its molar volume is found to be 5.00 × 10–4 m3 mol−1 at 273 K and 3.0 MPa. From this information calculate the van der Waals constant b. What is the compression factor for this gas at the prevailing temperature and pressure?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1


Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
A certain gas obeys the van der Waals equation with a = 0.50 m6 Pa mol−2. Its molar volume is found...
Questions






History, 23.09.2019 23:00

History, 23.09.2019 23:00



Mathematics, 23.09.2019 23:00

History, 23.09.2019 23:00

Mathematics, 23.09.2019 23:00




History, 23.09.2019 23:00


Mathematics, 23.09.2019 23:00

English, 23.09.2019 23:00

Mathematics, 23.09.2019 23:00

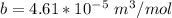
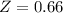
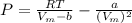
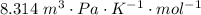 for R , 273K for T ,
for R , 273K for T , 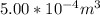 for
for 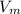 ,
, 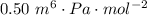 for a and
for a and 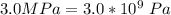 for P
for P