
Chemistry, 25.08.2020 01:01 HalpMahOnMahH0meW0rk
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate.
a)Calculate the volume of gas measured at r. t.p, produced in this reaction
b)calculatr the maximum mass of copper(ii)nitrate, produced in this reaction
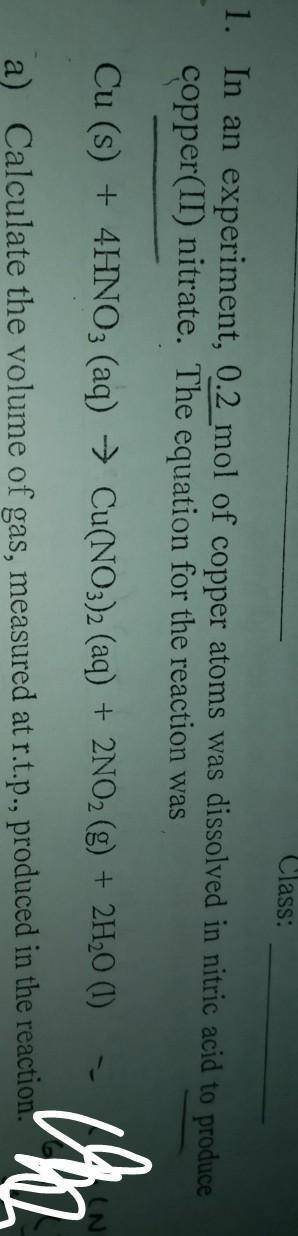

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate....
Questions

Mathematics, 11.01.2021 04:50

German, 11.01.2021 04:50




Mathematics, 11.01.2021 04:50

Mathematics, 11.01.2021 04:50



Mathematics, 11.01.2021 04:50




Mathematics, 11.01.2021 04:50



Mathematics, 11.01.2021 04:50


Mathematics, 11.01.2021 04:50

Mathematics, 11.01.2021 04:50



