
Chemistry, 12.08.2020 08:01 christabell0303
5. Two reactants combine to form a product in the reaction A + B -> C. The rate of the reaction depends on the concentrations of both
reactants squared (rate = k[A]2 [B]2). What's the total reaction order of this reaction?
A. 4
B. 1
C. 2.
O D.3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2


Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3
You know the right answer?
5. Two reactants combine to form a product in the reaction A + B -> C. The rate of the reaction d...
Questions



History, 14.07.2019 00:00


Social Studies, 14.07.2019 00:00






Biology, 14.07.2019 00:00



Mathematics, 14.07.2019 00:00

Mathematics, 14.07.2019 00:00





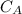 and
and 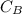 are the concentrations of A and B respectively at any time t, then assuming that the reaction is of first order with respect to both A and B , the overall order is second and the reaction rate is given by:
are the concentrations of A and B respectively at any time t, then assuming that the reaction is of first order with respect to both A and B , the overall order is second and the reaction rate is given by: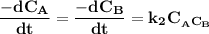
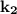 = specific rate constant for a second order reaction and becomes the rate of the reaction when both
= specific rate constant for a second order reaction and becomes the rate of the reaction when both 

