
Chemistry, 28.07.2020 05:01 FavvBella84
For the reaction C + 2H2 - CH2, how many moles of hydrogen are required to produce
19.26 mol of methane, CHA?
Select one:
O a. 19.26
O b. 38.52
O c. 15.0
O d. 24.7

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

You know the right answer?
For the reaction C + 2H2 - CH2, how many moles of hydrogen are required to produce
19.26 mol of met...
Questions



Mathematics, 04.11.2020 20:40



Mathematics, 04.11.2020 20:40

Spanish, 04.11.2020 20:40

Computers and Technology, 04.11.2020 20:40

Mathematics, 04.11.2020 20:40

Social Studies, 04.11.2020 20:40

English, 04.11.2020 20:40

Arts, 04.11.2020 20:40

Biology, 04.11.2020 20:40



Mathematics, 04.11.2020 20:40

English, 04.11.2020 20:40

History, 04.11.2020 20:40


Mathematics, 04.11.2020 20:40

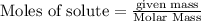
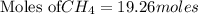
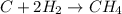
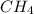 is produced by = 2 moles of
is produced by = 2 moles of 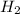
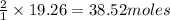 of
of 

