Consider the half reaction below.
Which statement best describes what is taking place?
...

Consider the half reaction below.
Which statement best describes what is taking place?
Chlorine is losing electrons and being oxidized. Chlorine is losing electrons and being reduced. Chlorine is gaining electrons and being oxidized. Chlorine is gaining electrons and being reduced.
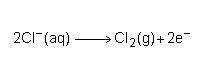

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

You know the right answer?
Questions

Mathematics, 30.11.2021 04:50

English, 30.11.2021 04:50

Business, 30.11.2021 04:50


Mathematics, 30.11.2021 04:50


Mathematics, 30.11.2021 04:50

History, 30.11.2021 04:50


Mathematics, 30.11.2021 04:50

Physics, 30.11.2021 04:50

SAT, 30.11.2021 04:50






Mathematics, 30.11.2021 04:50


Mathematics, 30.11.2021 04:50



