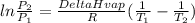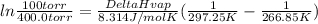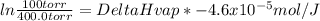Chemistry, 27.07.2020 01:01 ayoismeisalex
The vapor pressure of liquid chloroform, CHCl3, is 400.0 torr at 24.1 °C and 100.0 torr at –6.3 °C. What is DeltaH of vaporization for chloroform?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2


Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
You know the right answer?
The vapor pressure of liquid chloroform, CHCl3, is 400.0 torr at 24.1 °C and 100.0 torr at –6.3 °C....
Questions

Social Studies, 06.11.2020 19:30

Mathematics, 06.11.2020 19:30




Arts, 06.11.2020 19:30

Mathematics, 06.11.2020 19:30



Engineering, 06.11.2020 19:30


Mathematics, 06.11.2020 19:30



Mathematics, 06.11.2020 19:30


Mathematics, 06.11.2020 19:30



Social Studies, 06.11.2020 19:30