
Chemistry, 27.07.2020 01:01 ayoismeisjuam
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazoic acid is 2.20×10−5. Use the method of successive approximations in your calculations or the quadratic formula.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3



Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazo...
Questions

Mathematics, 30.07.2019 17:30

Mathematics, 30.07.2019 17:30





Social Studies, 30.07.2019 17:30

Business, 30.07.2019 17:30

Social Studies, 30.07.2019 17:30

Social Studies, 30.07.2019 17:30


History, 30.07.2019 17:30



History, 30.07.2019 17:30

Biology, 30.07.2019 17:30

History, 30.07.2019 17:30


Biology, 30.07.2019 17:30

![[H^+]=0.000285](/tpl/images/0713/5320/5d625.png)
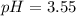
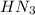 ). So:
). So:
![Ka=\frac{[H^+][N_3^-]}{[HN_3]}](/tpl/images/0713/5320/574a9.png)
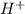 produced we will have 1 mol of
produced we will have 1 mol of 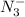 . So, we can use "X" for the unknown values and replace in the Ka equation:
. So, we can use "X" for the unknown values and replace in the Ka equation:![Ka=\frac{X*X}{[HN_3]}](/tpl/images/0713/5320/c6a9a.png)
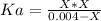
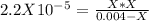
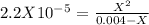
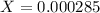
![pH=-Log[H^+]=-Log[0.000285]=3.55](/tpl/images/0713/5320/8cdec.png)


