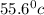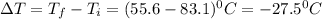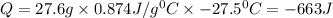Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
How much heat is released when 27.6 g P C l subscript 3 cools from 83.1 °C to 55.6 °C? (The specific...
Questions

Mathematics, 29.08.2019 08:30


Mathematics, 29.08.2019 08:30



Mathematics, 29.08.2019 08:30

Advanced Placement (AP), 29.08.2019 08:30


Geography, 29.08.2019 08:30



Mathematics, 29.08.2019 08:30


History, 29.08.2019 08:30

History, 29.08.2019 08:30

Health, 29.08.2019 08:30

History, 29.08.2019 08:30

Mathematics, 29.08.2019 08:30

Mathematics, 29.08.2019 08:30

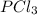 cools from 83.1 °C to 55.6 °C
cools from 83.1 °C to 55.6 °C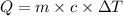
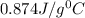
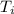 =
= 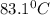
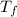 =
=