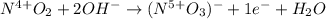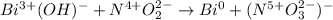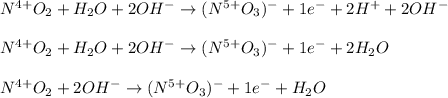Chemistry, 13.07.2020 21:01 lakiethalucas
The following skeletal oxidation-reduction reaction occurs under basic conditions. Write the balanced OXIDATION half reaction. Bi(OH)3 + NO2 → Bi + NO3-

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1


Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
The following skeletal oxidation-reduction reaction occurs under basic conditions. Write the balance...
Questions

Mathematics, 13.11.2020 20:30


Mathematics, 13.11.2020 20:30

Mathematics, 13.11.2020 20:30


Mathematics, 13.11.2020 20:30

Chemistry, 13.11.2020 20:30

Mathematics, 13.11.2020 20:30

Chemistry, 13.11.2020 20:30



Mathematics, 13.11.2020 20:30







Chemistry, 13.11.2020 20:30

History, 13.11.2020 20:30