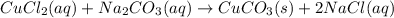Mixing which 0.1 M aqueous solutions results in formation of a colored precipitate? A) BaCl2 and CH3COOH (B) BaCl2 and Na2CO3 (C) CuCl2 and CH3COOH (D) CuCl2 and Na2CO3 The correct answer is D but please explain I'm confused. I know that the product has to be insoluble to form a precipitate, but how do you know if it'll be colored?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
Mixing which 0.1 M aqueous solutions results in formation of a colored precipitate? A) BaCl2 and CH3...
Questions



Mathematics, 02.04.2020 02:14

Biology, 02.04.2020 02:14


Mathematics, 02.04.2020 02:14


English, 02.04.2020 02:14



Mathematics, 02.04.2020 02:15


English, 02.04.2020 02:15

History, 02.04.2020 02:15


Arts, 02.04.2020 02:15