Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
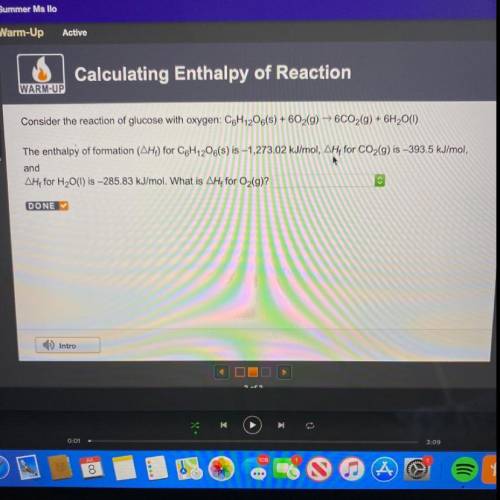
Consider the reaction of glucose with oxygen: C6H12O6(s) + 602(g) → 6CO2(g) + 6H2O(1)
The enthalpy...
Questions


Social Studies, 10.12.2021 19:20




Mathematics, 10.12.2021 19:20

Mathematics, 10.12.2021 19:20


Mathematics, 10.12.2021 19:20



History, 10.12.2021 19:20

Mathematics, 10.12.2021 19:20


English, 10.12.2021 19:20



Mathematics, 10.12.2021 19:20