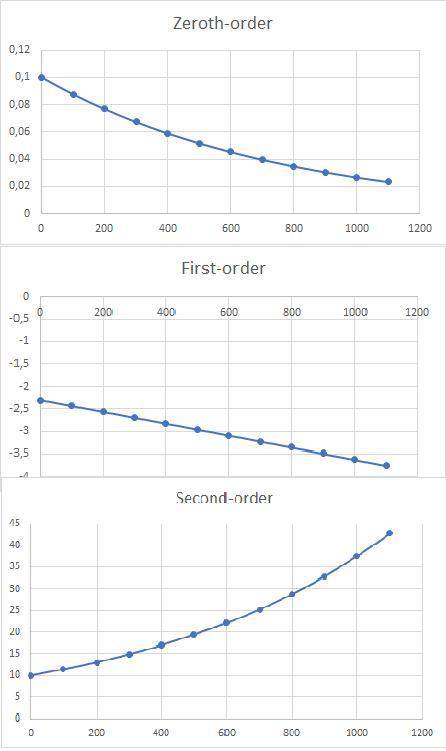
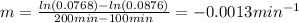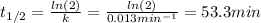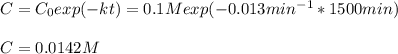3.The following data of decomposition reaction of thionyl chloride (SO2Cl2) were collected at a certain temperature and the concentration of SO2Cl2 were monitored as shown in the table.
SO2Cl2 (g) SO2 (g) + Cl2 (g)
Time (min)Conc. of SO2Cl2 (mol/L)
00.1000
1000.0876
2000.0768
3000.0673
4000.0590
5000.0517
6000.0453
7000.0397
8000.0348
9000.0305
10000.0267
11000.0234
a)Determine graphically whether the kinetics of the reaction is zero order, first order or second order with respect to SO2Cl2 and then write the rate equation.
b)Determine the rate constant (k) of the reaction.
c)Determine the half-life (t½) for the reaction.
d)What will be the concentration of SO2Cl2 left in the reaction mixture at 1500 minutes?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
3.The following data of decomposition reaction of thionyl chloride (SO2Cl2) were collected at a cert...
Questions

Chemistry, 06.01.2021 01:40



English, 06.01.2021 01:40

Biology, 06.01.2021 01:40






Engineering, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40


History, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40

English, 06.01.2021 01:40

Computers and Technology, 06.01.2021 01:40