
In E. coli, the enzyme hexokinase catalyzes the reaction: Glucose + ATP → glucose 6-phosphate + ADP The equilibrium constant, Keq, is 7.8 x 102. In the living E. coli cells, [ATP] = 7.9 mM; [ADP] = 1.04 mM, [glucose] = 2 mM, [glucose 6-phosphate] = 1 mM. Determine if the reaction is at equilibrium. If the reaction is not at equilibrium, determine which side the reaction favors in living E. coli cells.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
On a distance vs time graph the line of an object at rest is a
Answers: 1

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

You know the right answer?
In E. coli, the enzyme hexokinase catalyzes the reaction: Glucose + ATP → glucose 6-phosphate + ADP...
Questions

Spanish, 14.01.2021 04:00

Physics, 14.01.2021 04:00

Arts, 14.01.2021 04:00



Mathematics, 14.01.2021 04:00

Mathematics, 14.01.2021 04:00

Mathematics, 14.01.2021 04:00



Social Studies, 14.01.2021 04:00




English, 14.01.2021 04:00


English, 14.01.2021 04:00

Biology, 14.01.2021 04:00

Spanish, 14.01.2021 04:00

English, 14.01.2021 04:00

![q=\frac{[\text {glucose 6-phosphate}][ADP]}{[Glucose][ATP]}](/tpl/images/0683/3692/46a36.png)
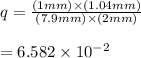
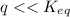 ⇒ following this criteria the reaction will go towards the right direction ( that is forward reaction is favorable until q = Keq
⇒ following this criteria the reaction will go towards the right direction ( that is forward reaction is favorable until q = Keq

