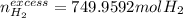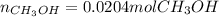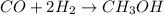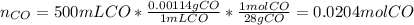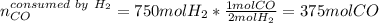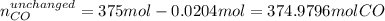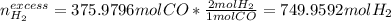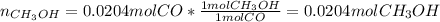Chemistry, 05.06.2020 16:00 nxusasmangaliso1191
Methanol CH3OH is the simplest of the alcohols . It is sinthesized by the reaction of hydrogen and carbon monoxide.
CO + H2 > CH3OH
a) If 500 ml CO and 750 mol H2 are present, which is the limiting reactant?
b) How many mols of excess reactant remain unchange?
c) How many moles of CH3OH are formed ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1
You know the right answer?
Methanol CH3OH is the simplest of the alcohols . It is sinthesized by the reaction of hydrogen and c...
Questions


Mathematics, 24.10.2019 17:43




Physics, 24.10.2019 17:43

Mathematics, 24.10.2019 17:43


Mathematics, 24.10.2019 17:43



English, 24.10.2019 17:43


Mathematics, 24.10.2019 18:43

Mathematics, 24.10.2019 18:43

English, 24.10.2019 18:43


Chemistry, 24.10.2019 18:43

Mathematics, 24.10.2019 18:43