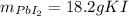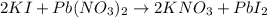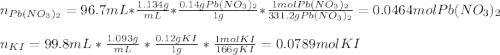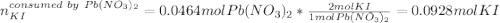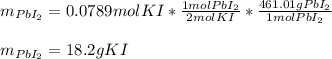Chemistry, 05.06.2020 16:00 mirandaperez3412
1.A 99.8 mL sample of a solution that is 12.0% KI by mass (d: 1.093 g/mL) is added to 96.7 mL of another solution that is 14.0% Pb(NO3)2 by mass (d: 1.134 g/mL). How many grams of PbI2 should form? Pb(NO3)2(aq) + 2 KI(aq) PbI2(s) + 2 KNO3(aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1

Chemistry, 23.06.2019 14:00
During an acid-base titration, when do the contents of the beaker consist of only water, a salt, and a trace of indicator?
Answers: 2

Chemistry, 23.06.2019 15:30
How is the electron sea model of metallic bonding different from the band theory? how are they the same? give at least one similarity and one difference between the models
Answers: 3
You know the right answer?
1.A 99.8 mL sample of a solution that is 12.0% KI by mass (d: 1.093 g/mL) is added to 96.7 mL of ano...
Questions

Geography, 20.02.2020 01:59



Computers and Technology, 20.02.2020 01:59










History, 20.02.2020 01:59

Mathematics, 20.02.2020 01:59


Business, 20.02.2020 01:59


Business, 20.02.2020 01:59