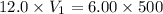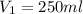Chemistry, 05.06.2020 08:59 rmcarde3453
You are required to prepare 500 ml of a 6.00 M solution of HNO3 from a stock solution of 12.0 M. Describe in detail how you would go about preparing this solution. Clearly state the volume of stock solution used, the glassware's used and the procedure.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Assume that the variables x and y are inversely related. if k = 18, what is the value of y for each of the following points? be sure and record your data to be used in the following problem.
Answers: 2


Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
You are required to prepare 500 ml of a 6.00 M solution of HNO3 from a stock solution of 12.0 M. Des...
Questions



Mathematics, 19.04.2020 00:16






Mathematics, 19.04.2020 00:16




Mathematics, 19.04.2020 00:16


Mathematics, 19.04.2020 00:16

Mathematics, 19.04.2020 00:16

English, 19.04.2020 00:16



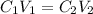
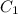 = concentration of stock solution = 12.0 M
= concentration of stock solution = 12.0 M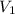 = volume of stock solution = ?
= volume of stock solution = ?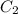 = concentration of diluted solution= 6.00 M
= concentration of diluted solution= 6.00 M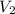 = volume of diluted acid solution = 500 ml
= volume of diluted acid solution = 500 ml