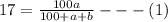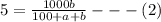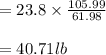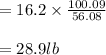Soda and lime are added to a glass batch in the form of soda ash (Na2CO3 ) and lime-stone (CaCO3 ). During heating, these two ingredients decompose to give off carbon dioxide (CO2 ), the resulting products being soda and lime. Compute the weight of soda ash and limestone that must be added to 125 lbm of quartz (SiO2 ) to yield a glass of composition 78 wt% SiO2 , 17 wt% Na2O, and 5 wt% CaO.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1



You know the right answer?
Soda and lime are added to a glass batch in the form of soda ash (Na2CO3 ) and lime-stone (CaCO3 )....
Questions

Mathematics, 26.08.2020 07:01


Social Studies, 26.08.2020 07:01


Mathematics, 26.08.2020 07:01





Chemistry, 26.08.2020 07:01

Mathematics, 26.08.2020 07:01



English, 26.08.2020 07:01

Mathematics, 26.08.2020 07:01



Mathematics, 26.08.2020 07:01

Mathematics, 26.08.2020 07:01