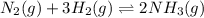Chemistry, 22.05.2020 03:05 mcmccann4317
Explain why it is necessary to balance chemical equations in terms of maintaining equilibrium. How can you tell when a chemical reaction has reached equilibrium? Give an example to help explain your answer.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
Explain why it is necessary to balance chemical equations in terms of maintaining equilibrium. How c...
Questions



Physics, 28.08.2019 04:00


Biology, 28.08.2019 04:00

Physics, 28.08.2019 04:00

English, 28.08.2019 04:00

Mathematics, 28.08.2019 04:00


Social Studies, 28.08.2019 04:00


Chemistry, 28.08.2019 04:00



Biology, 28.08.2019 04:00


Mathematics, 28.08.2019 04:00