20 POINTS AND GIVING BRAINLIEST
The following reaction shows the products when sulfuric a...

Chemistry, 22.05.2020 01:05 baileyflemingde
20 POINTS AND GIVING BRAINLIEST
The following reaction shows the products when sulfuric acid and aluminum hydroxide react.
2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H2O
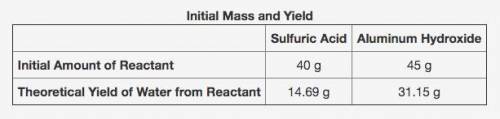
The table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. (See attached image)
What is the approximate amount of the leftover reactant?
A) 20.89 g of sulfuric acid
B) 22.44 g of sulfuric acid
C) 21.22 g of aluminum hydroxide
D) 23.78 g of aluminum hydroxide

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:50
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Questions

Mathematics, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00

History, 04.02.2021 16:00


Mathematics, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00

Chemistry, 04.02.2021 16:00

English, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00



Mathematics, 04.02.2021 16:00

English, 04.02.2021 16:00



World Languages, 04.02.2021 16:00

Computers and Technology, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00

Chemistry, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00



